| THE FUNDAMENTAL CONCEPTS - ELEMENTS, COMPOUNDS, MOLECULES, AND ATOMS | ||
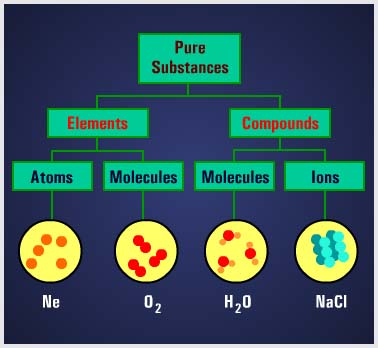
An ELEMENT is a substance that can’t be reduced to a simpler substance by chemical means. Examples of elements are iron, gold, silver, copper, and oxygen. When two or more elements are combined, the result is a COMPOUND. Examples of common compounds are water, which consists of hydrogen and oxygen, and table salt, which consists of sodium and chlorine. A MOLECULE is a chemical combination of two or more atoms. In a compound, the molecule is the smallest particle that has all the characteristics of the compound. Molecules in turn are made up of smaller particles called ATOMS. An atom is the smallest particle of an element that retains the characteristics of that element. |
|
|
| 3 of 67 | ||