| THE FUNDAMENTAL CONCEPTS - ATOMIC STRUCTURE | ||
The atoms of each element are made up of electrons, protons, and, in most cases, neutrons, and are collectively called subatomic particles. The electron is considered to be a small negative charge of electricity. The proton has a positive charge of electricity equal and opposite to the charge of the electron. Scientists know the mass and size of the electron and proton, and how much charge each possesses. Both have the same quantity of charge, although the mass of the proton is many times that of the electron. In most atoms, there exists a neutral particle called a neutron. The neutron has a mass about equal to that of a proton, but it has no electrical charge. |
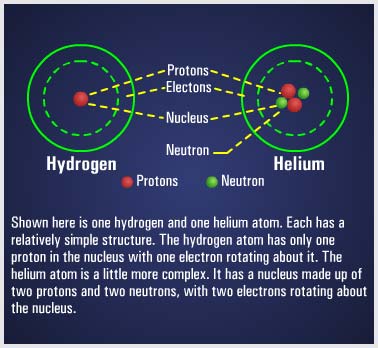
|
|
| 4 of 67 | ||