| THE FUNDAMENTAL CONCEPTS - ENERGY LEVELS | ||
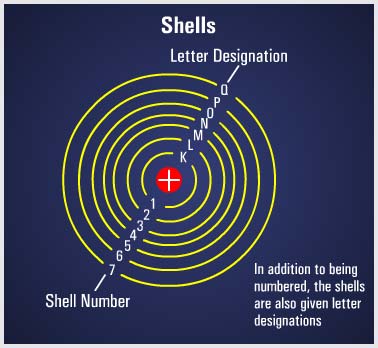
It must be emphasized that the electron cannot jump to just any orbit. The electron will remain in its lowest orbit until a sufficient amount of energy is available, at which time the electron will accept the energy and jump to one of a series of permissible orbits. Heat energy and collisions with other particles can also cause the electron to jump orbits. In general, the electrons reside in groups of orbits called shells. These shells are elliptically shaped and are assumed to be located at fixed intervals. Thus, the shells are arranged in steps that correspond to fixed energy levels. |
|
|
| 9 of 67 | ||