| THE FUNDAMENTAL CONCEPTS - VALENCE ELECTRONS | ||
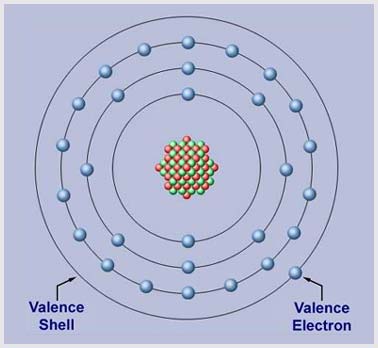
The number of electrons in the outermost shell determines the valence of an atom. For this reason, the outer shell of an atom is called the VALENCE SHELL; and the electrons in this shell are called VALENCE ELECTRONS. An atom’s valence determines its ability to gain or lose an electron, which in turn determines the chemical and electrical properties of the atom. As we shall see next, it is the valence electrons that we are most concerned with in electricity. |
|
|
| 10 of 67 | ||